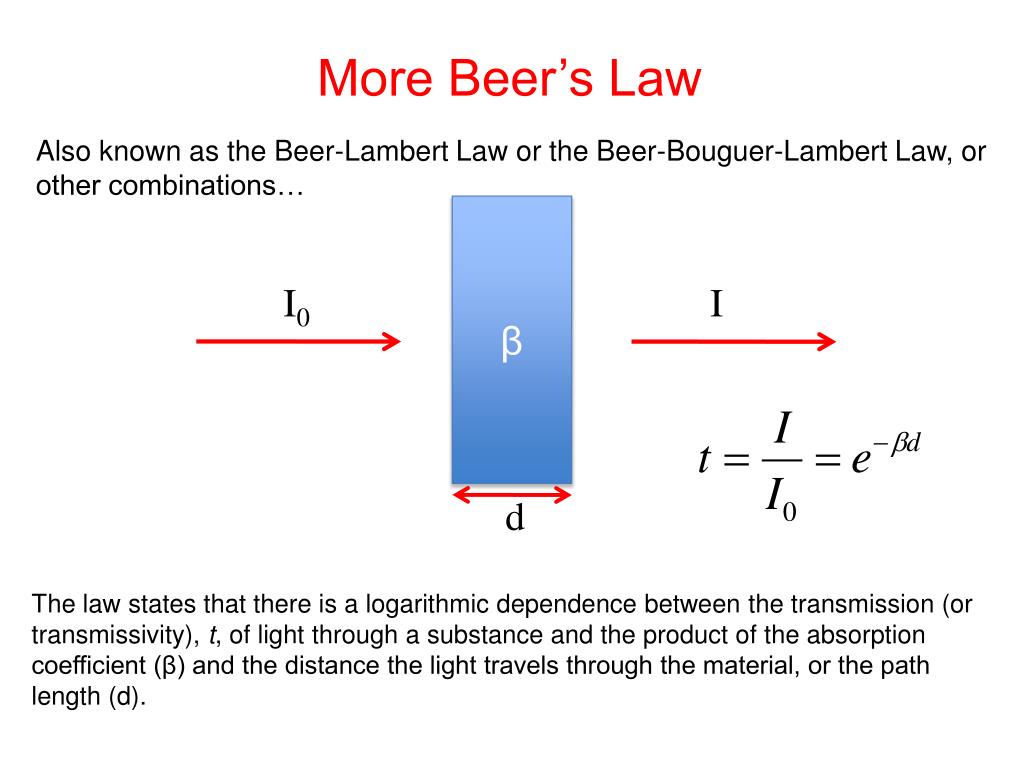
Beer Lambert S Law States That . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer's law states that the absorbance is proportional to the concentration of the sample. Technically, beer's law relates only to. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless.
from www.slideserve.com
beer's law states that the absorbance is proportional to the concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. Technically, beer's law relates only to. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
PPT Atmospheric Transmission PowerPoint Presentation, free download
Beer Lambert S Law States That beer's law states that the absorbance is proportional to the concentration of the sample. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. Technically, beer's law relates only to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. beer's law states that the absorbance is proportional to the concentration of the sample.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert S Law States That It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer Lambert S Law States That.
From dxowmggun.blob.core.windows.net
LambertBeer's Law Was Described To A General Equation at Thomas Partin Beer Lambert S Law States That In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer's law states that the absorbance is proportional to the concentration of the sample. Technically, beer's law relates only to. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Beer Lambert S Law States That.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert S Law States That It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. in spectroscopy,. Beer Lambert S Law States That.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert S Law States That It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. in spectroscopy,. Beer Lambert S Law States That.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert S Law States That In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length. Beer Lambert S Law States That.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert S Law States That Technically, beer's law relates only to. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer's law states that the absorbance is proportional to the concentration of the sample.. Beer Lambert S Law States That.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert S Law States That Technically, beer's law relates only to. beer's law states that the absorbance is proportional to the concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its. Beer Lambert S Law States That.
From www.slideserve.com
PPT chapter 2 PowerPoint Presentation, free download ID540228 Beer Lambert S Law States That in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. It provides a mathematical relationship between the substance’s concentration in a solution. Beer Lambert S Law States That.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer Lambert S Law States That Technically, beer's law relates only to. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\). Beer Lambert S Law States That.
From www.youtube.com
General Application of Beerlambert law YouTube Beer Lambert S Law States That Technically, beer's law relates only to. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer lambert’s law states that. Beer Lambert S Law States That.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert S Law States That It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Technically, beer's law relates only to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more. Beer Lambert S Law States That.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert S Law States That In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional. Beer Lambert S Law States That.
From www.slideserve.com
PPT Lab 6 Saliva Practical BeerLambert Law PowerPoint Presentation Beer Lambert S Law States That In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. Technically, beer's law relates only to. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\]. Beer Lambert S Law States That.
From www.youtube.com
Numerical based on Beer Lambert's Law Simple and easy calculations Beer Lambert S Law States That beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it. Beer Lambert S Law States That.
From dxoccvohi.blob.core.windows.net
Beer's Law Path Length at John Lee blog Beer Lambert S Law States That It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer's law states that the absorbance is proportional to the concentration of the sample. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer lambert’s law states. Beer Lambert S Law States That.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert S Law States That in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is. Beer Lambert S Law States That.
From www.youtube.com
Beer lamberts law (Optics lab.) YouTube Beer Lambert S Law States That beer lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more monochromatic light the further it passes. Beer Lambert S Law States That.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert S Law States That \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Technically, beer's law relates only to. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the. Beer Lambert S Law States That.